- Physical properties of a gas - gases heat up during compression -
For pressure tests on gas pipelines, which are carried out with air or nitrogen, there are some physical effects, which we will try to explain here in a very simple way.
Physical properties of gas:
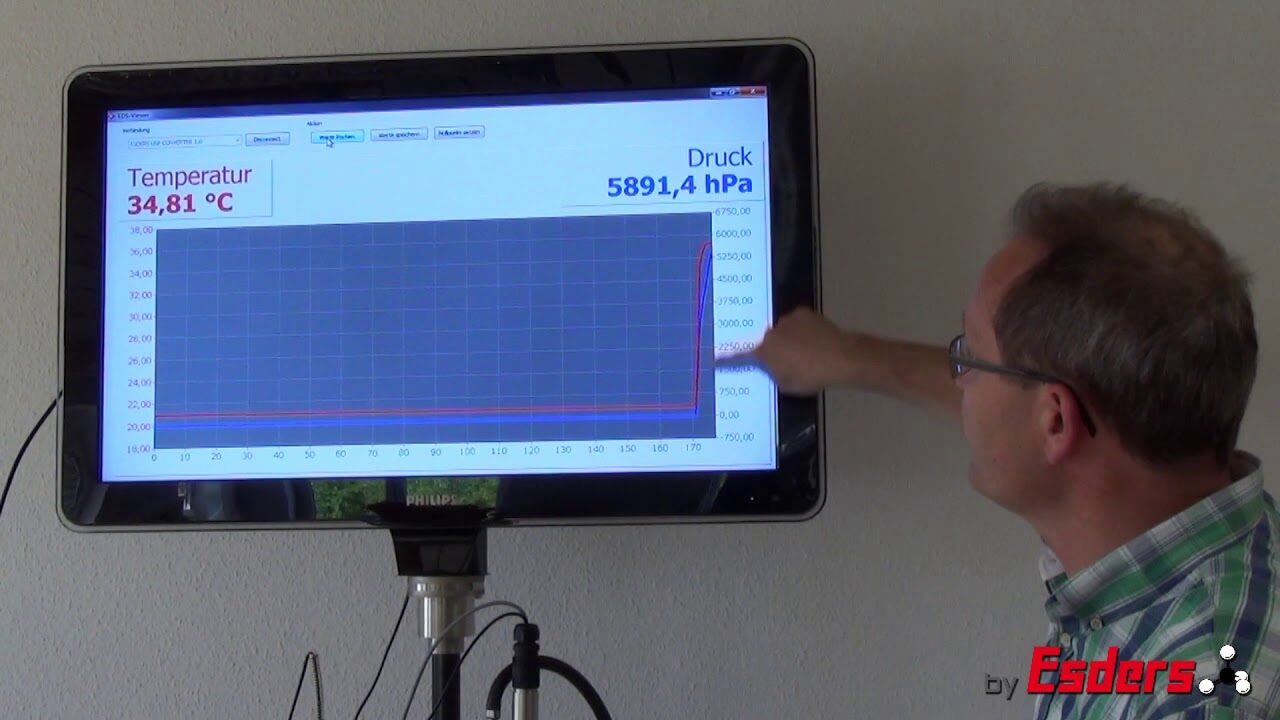
If the pressure in a pipe is increased rapidly, there is always a rise in temperature. Gases heat up during compression. Everyone has pumped up a bicycle tire with an air pump. Even then, the increase in temperature of the pump can be felt very clearly. By compressing air, the temperature of the air increases.
Principles that are helpful for evaluating tests in practice:
Pressurisation of a pipeline always leads to an increase in temperature due to the compression of the air. This temperature rise should be compensated for by a stabilisation period. For the pressure test, it is important to note that after pressurisation, the increased temperature slowly adjusts to the ambient temperature again. However, this falling temperature also leads to a reduction/drop in pressure. This drop in pressure is not due to leakage. For this reason, we have to wait for an adaptation period after pressurisation before starting the actual pressure test, so that a pressure drop due to temperature is not included in the pressure test.
A special case is the pressure test on plastic pipes.
Video: Applying pressure
Pressure drop due to expansion: Plastic pipes expand under pressure. This leads to a drop in pressure.
Several effects occur during pressure testing on plastic pipes such as polyethylene (PE) pipes. The temperature is increased by compressing air or nitrogen. This temperature then adjusts to the ambient temperature and it results in a pressure drop in the pipeline. But a second effect occurs. Plastic pipes expand under pressure. This effect also takes some time and leads to a pressure drop.
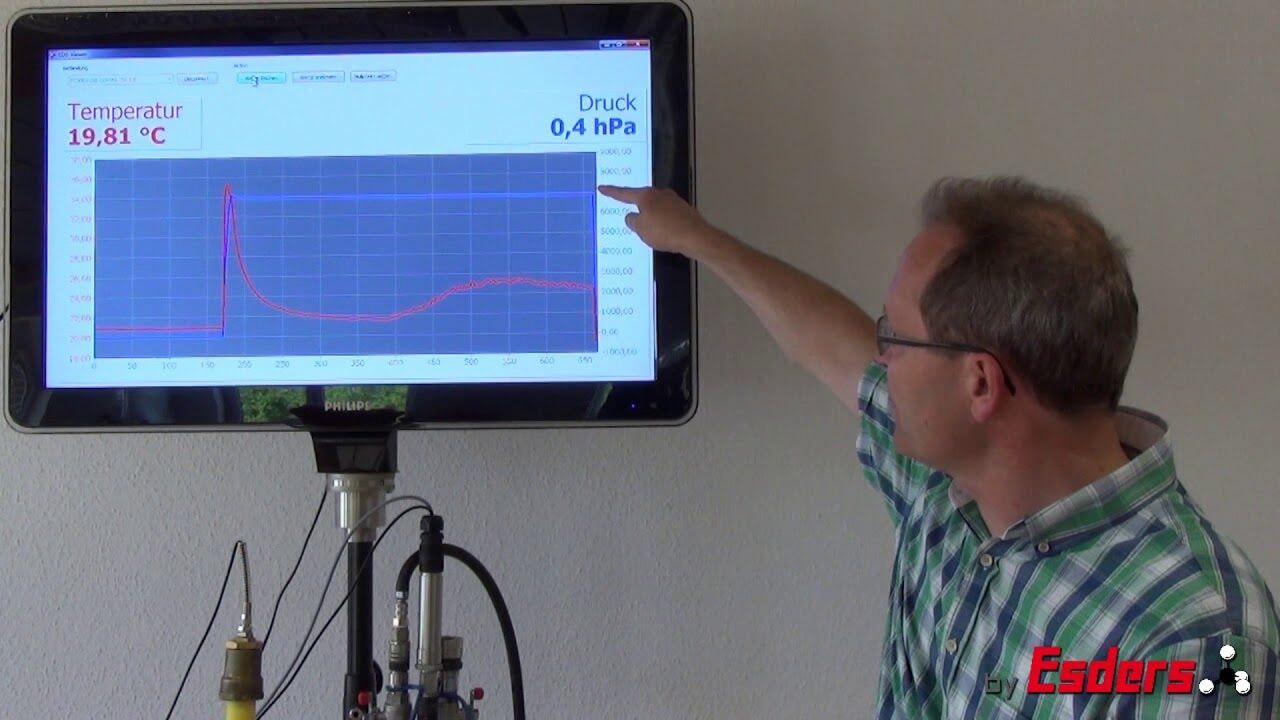
Video: Cooling due to expansion
In addition to the warming due to compression of the air in the pipeline, a similar effect occurs, the cooling during expansion. This can be important if a load test/strength test at higher pressures is carried out first and then a tightness test at lower pressure is added.
When air expands, there is a pressure drop which also has a direct influence on the temperature, which falls in the process. The effect is the opposite of that of compressing air. Once again, an adaptation period should be observed for a tightness test, which should be carried out after a strength test in the higher pressure range.
In a second article on pressure tests on gas pipelines in our blog, we will once again deal with pressure reduction and situations from everyday working life, such as the influence of the sun on the results of the pressure test and what needs to be taken into account.